Cryptate Chemistry
Good FRET pairs exhibit no overlapping spectrum between donor and acceptor, high quantum yields, and red-shifted emission to minimize compound interference. The best donors are cryptates, comprised of rare-earth complexes (europium or terbium) with a lanthanide embedded in a macrocycle1. They exhibit long-lived fluorescence, stability, and robustness necessary to survive many assay conditions. South Bay Bio offers time-tested europium (and soon terbium cryptates) comprised of a tris-bipyridine macrocycle encasing the lanthanide ion, and the most common linker is maleimide.
Acceptor Fluorophores
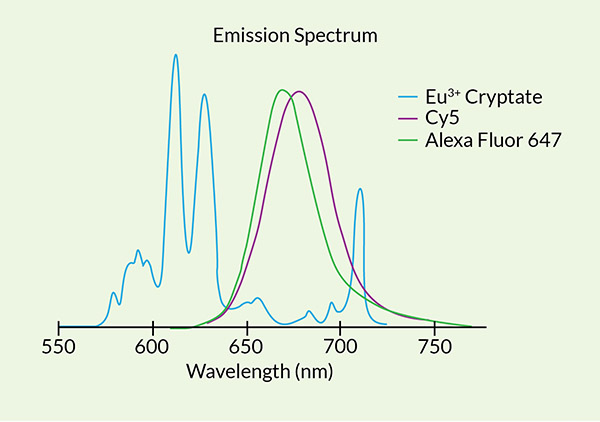
Several acceptor fluorophores work well for FRET pairing with europium and terbium cryptate donor. Cy5 and Alexa Fluor 647 optimally match excitation spectra with emission profiles of the europium donor, and are detected in the far-red range of the spectrum. Terbium based donors can be paired with FITC in the green spectrum. These acceptors exhibit low levels of non-specific binding to biomolecules, large extinction coefficients, large quantum yields, and bright fluorescence2. Other fluorophores can also be used if they better suit your needs. We are also developing a Samarium based system.
References
1) Lehn, Jean Marie. "Cryptates: The Chemistry of Macropolycyclic Inclusion Complexes." Accounts of Chemical Research 11.2 (1978): 49-57.
2) Iqbal, Asif, Lihua Wang, Katherine C. Thompson, David M. J. Lilley, and David G. Norman. "The Structure of Cyanine 5 Terminally Attached to Double-Stranded DNA: Implications for FRET Studies." Biochemistry 47.30 (2008): 7857-862.
